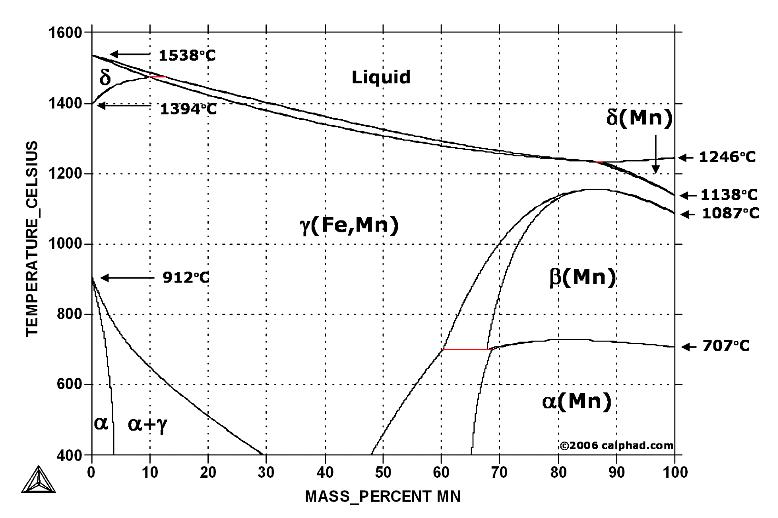
In pure iron, the A4 (1394 °C) and A3 (912 °C) transformations take place at constant temperatures. If an element enters into solid solution in iron — forming in that way a binary alloy — each of these transformations are required by the Phase Rule to occur over a range of temperature. Some elements, such as manganese, raise the A4 and lower the A3 transformation temperatures below increasing, in effect, the extent of the gamma field in the iron-carbon phase diagram.
Fe-Mn phase diagram shows which phases are to be expected at equilibrium for different combinations of manganese content and temperature. The melting point of iron and manganese at the pressure of 101325 Pa is 1538 °C and 1246 °C, respectively.
You might also like
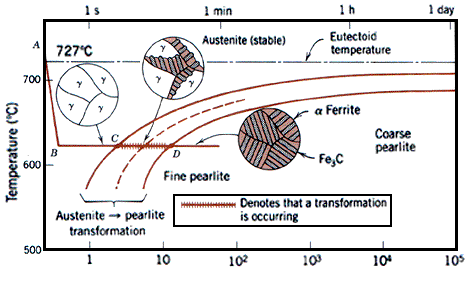
| Fe-Fe3C T-T-T Diagram Fe-Fe3C T-T-T Diagram, Adapted from... | Phase Diagram of Steel Fe-Fe3C Phase Diagram, Materials Science... | Bainite Bainite is an acicular microstructure... | Metallurgy Glossary Metallurgy Glossary Activity: A function... |

 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope