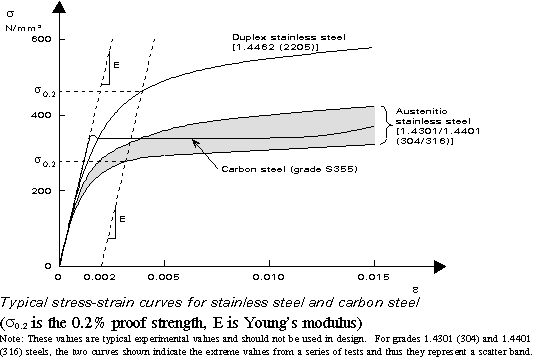
According to European standard EN 10 020, steel is a material which contains by weight more iron than any other single element, having a carbon content generally less than 2% and containing other elements (Figure 1). A limited number of chromium steels may contain more than 2% of carbon, but 2% is the usual dividing line between steel and cast iron.
 Steel Wire
Steel Wire
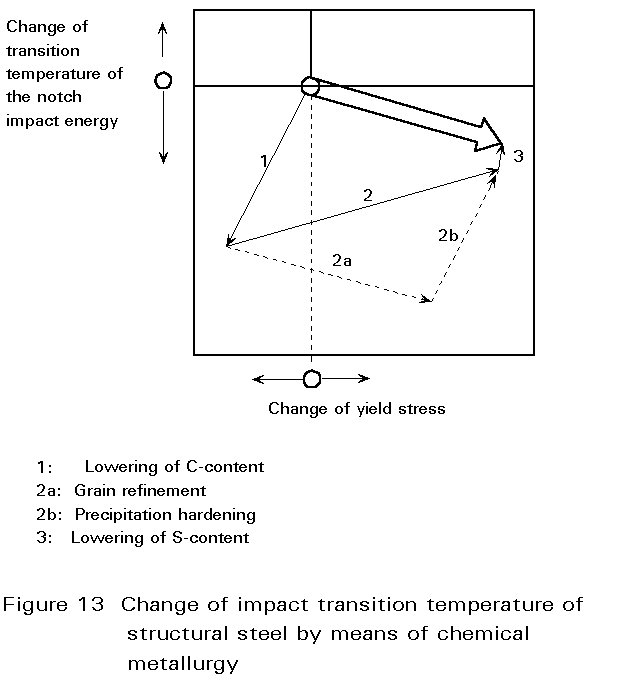
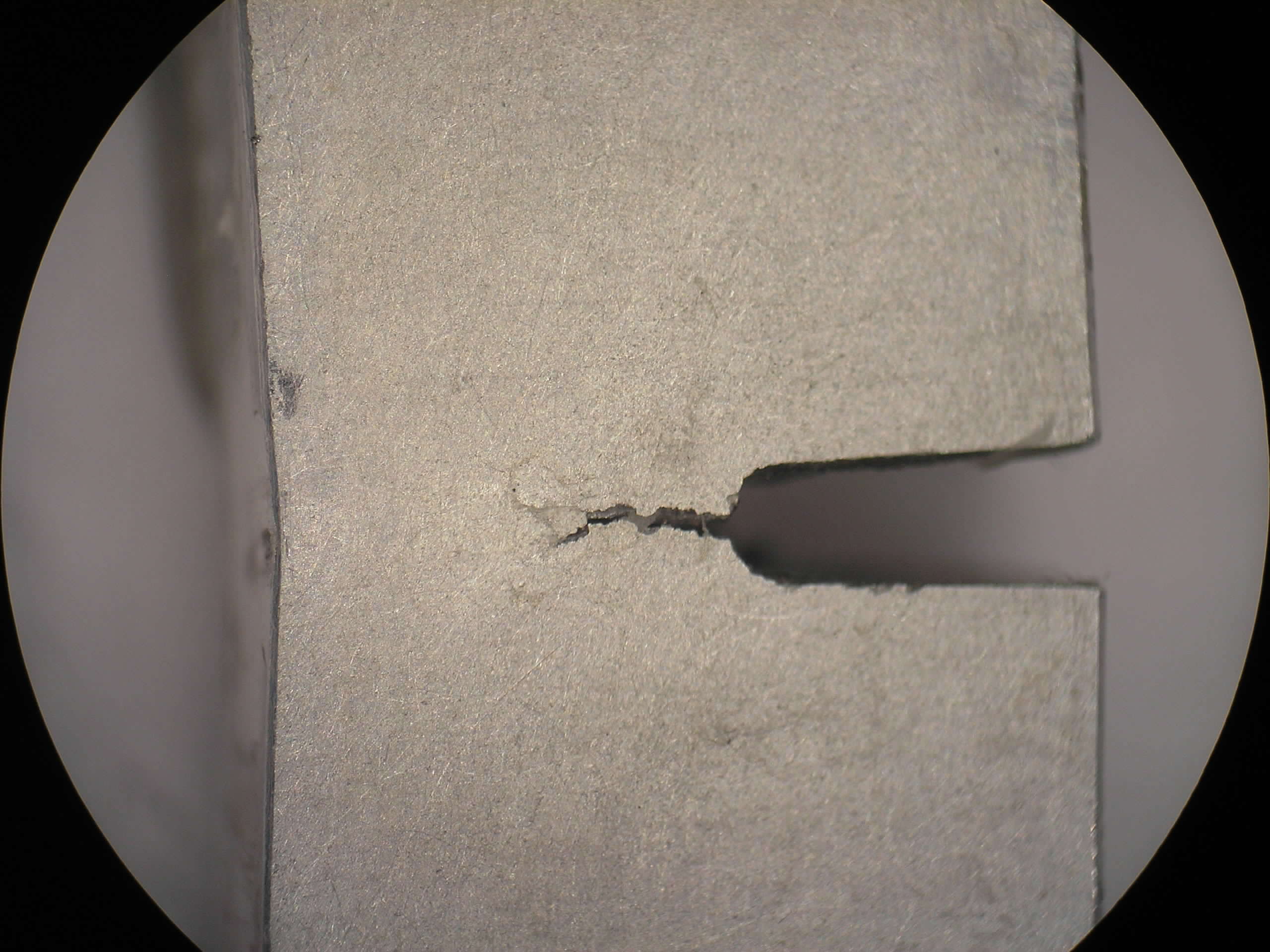
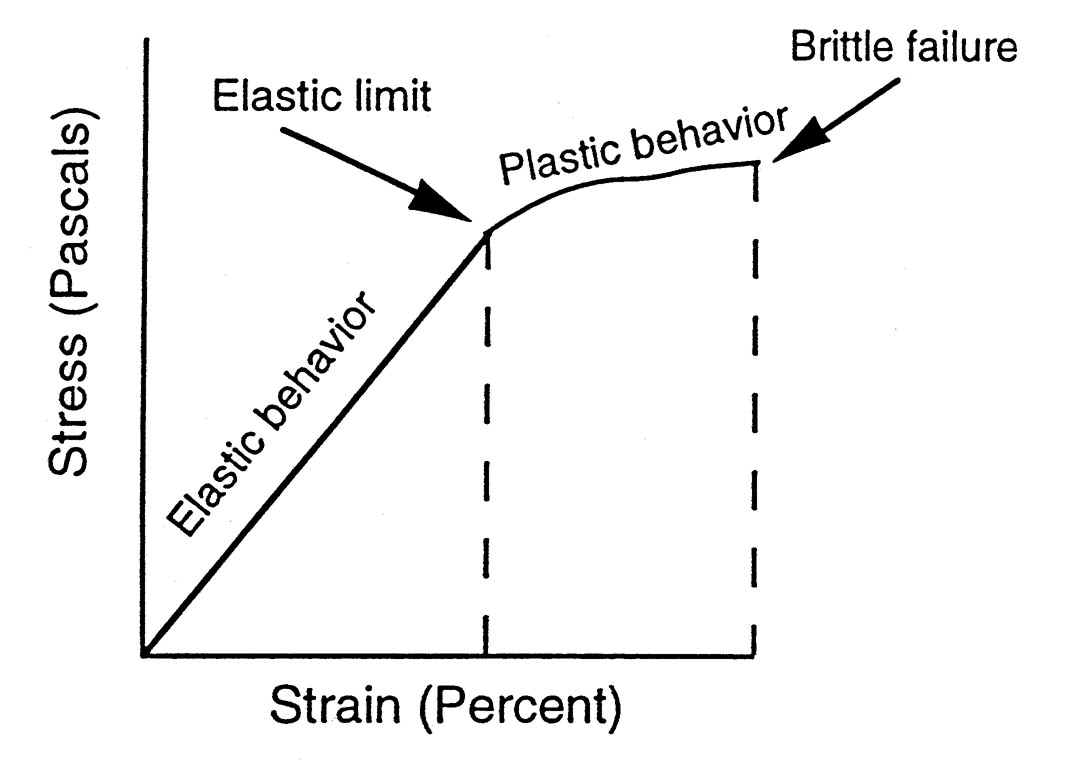
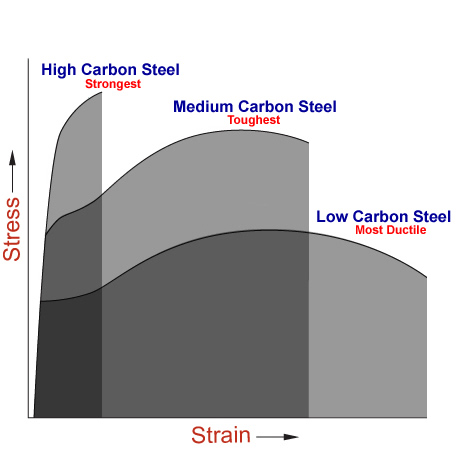
Due to its high strength, its good machineability and its high economic efficiency, steel is one of the most important construction materials. By changes in the chemical composition and in the production conditions, it is possible to vary steel properties over a wide range and the steel manufacturer is able to adapt the properties to the specific requirements of users.
You might also like
| Optimal Combination of STRENGTH and TOUGHNESS Preceding sections have described the influence... | Failure analysis Failure analysis is the process of... | Austempered Ductile Iron (ADI) Austempered Ductile Iron (ADI) is... | Fracture Mechanics Concepts The basis of a fracture mechanics safety... |







 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope