What is Cast Iron?
Cast irons typically contain 2-4 wt% of carbon with a high silicon concentrations and a greater concentration of impurities than steels. The carbon equivalent (CE) of a cast iron helps to distinguish the grey irons which cool into a microstructure containing graphite and and the white irons where the carbon is present mainly as cementite. The carbon equivalent is defined as:
![]()
A high cooling rate and a low carbon equivalent favours the formation of white cast iron whereas a low cooling rate or a high carbon equivalent promotes grey cast iron.
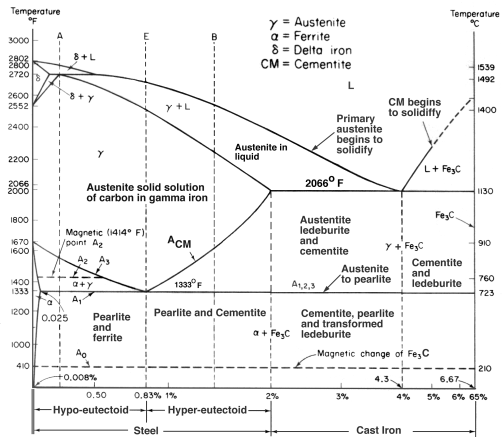 FeC Phasae Diagram
FeC Phasae Diagram
During solidification, the major proportion of the carbon precipitates in the form of graphite or cementite. When solidification is just complete, the precipitated phase is embedded in a matrix of austenite which has an equilibrium carbon concentration of about 2 wt%. On further cooling, the carbon concentration of the austenite decreases as more cementite or graphite precipitates from solid solution. For conventional cast irons, the austenite then decomposes into pearlite at the eutectoid temperature. However, in grey cast irons, if the cooling rate through the eutectoid temperature is sufficiently slow, then a completely ferritic matrix is obtained with the excess carbon being deposited on the already existing graphite. White cast irons are hard and brittle, they cannot easily be machined.
Cast iron is made by re-melting pig iron, often along with substantial quantities of scrap iron and scrap steel and taking various steps to remove undesirable contaminants such as phosphorus and sulphur. Depending on the application, carbon and silicon content are reduced to the desired levels, which may be anywhere from 2 to 3.5% and 1 to 3% respectively. Other elements are then added to the melt before the final form is produced by casting. Iron is sometimes melted in a special type of blast furnace known as a cupola, but more often melted in electric induction furnaces. After melting is complete, the molten iron is poured into a holding furnace or ladle.
 Cast Iron Pulley
Cast Iron Pulley
Phosphorus and sulphur are undesirable elements to have in iron and steel and need to be burnt out. Unfortunately the desirable carbon burns away first so the carbon then has to be replaced. Allegedly the cheapest steel as used by budget quality car manufacturers has the sulphur and phosphorus left in. This latter allegation will be very much a trade secret, however the evidence of excessive corrosion can be found in scrapyards all over the world. Some steels known as “weathering steels” hardly rust at all.
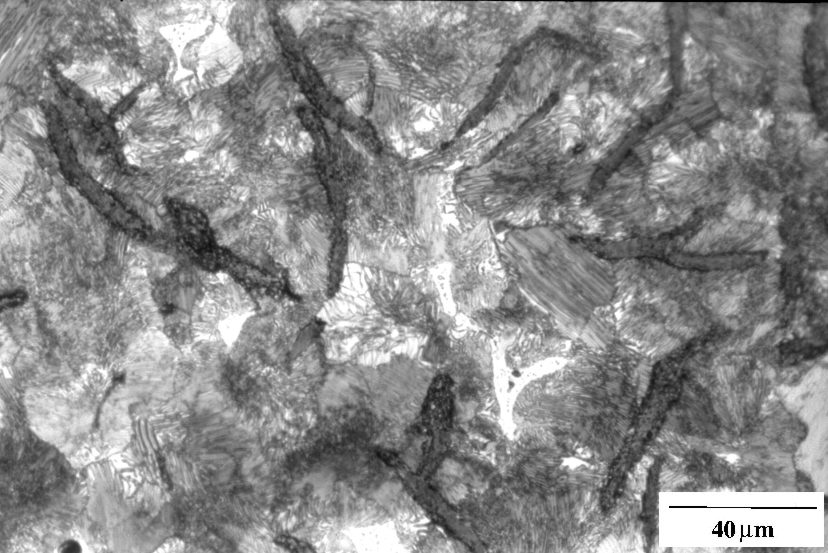 Grey cast iron, Fe-3.2C-2.5Si wt%, containing graphite flakes in a matrix which is pearlitic. The lamellar structure of the pearlite can be resolved, appearing to consist of alternating layers of cementite and ferrite. The speckled white regions represent a phosphide eutectic. Etchant: Nital 2%
Grey cast iron, Fe-3.2C-2.5Si wt%, containing graphite flakes in a matrix which is pearlitic. The lamellar structure of the pearlite can be resolved, appearing to consist of alternating layers of cementite and ferrite. The speckled white regions represent a phosphide eutectic. Etchant: Nital 2%
Before they came to be made of mild steel, items produced from wrought iron included rivets, nails, wire, chains, railway couplings, water and steam pipes, nuts, bolts, horseshoes, handrails, straps for timber roof trusses, and ornamental ironwork. Wrought iron is no longer produced on a commercial scale. Many products described as wrought iron, such as guard rails, garden furniture and gates, are made of mild steel. They retain that description because they are wrought (worked) by hand.
Grey cast iron
Grey cast iron is characterized by its graphitic microstructure, which causes fractures of the material to have a grey appearance. It is the most commonly used cast iron and the most widely used cast material based on weight. Most cast irons have a chemical composition of 2.5 to 4.0% carbon, 1 to 3% silicon, and the remainder is iron.
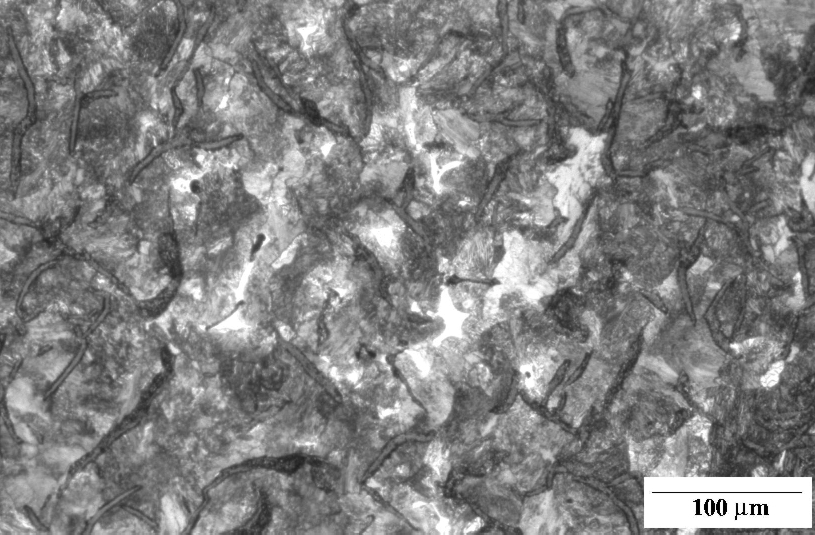 Grey cast iron, Fe-3.2C-2.5Si wt%, containing graphite flakes in a matrix which is pearlitic. The speckled white regions represent a phosphide eutectic. Etchant: Nital 2%
Grey cast iron, Fe-3.2C-2.5Si wt%, containing graphite flakes in a matrix which is pearlitic. The speckled white regions represent a phosphide eutectic. Etchant: Nital 2%
Grey cast iron has less tensile strength and shock resistance than steel, but its compressive strength is comparable to low and medium carbon steel.
White cast iron
With a lower silicon content and faster cooling, the carbon in white cast iron precipitates out of the melt as the metastable phase cementite, Fe3C, rather than graphite. The cementite which precipitates from the melt forms as relatively large particles, usually in a eutectic mixture, where the other phase is austenite (which on cooling might transform to martensite). These eutectic carbides are much too large to provide precipitation hardening (as in some steels, where cementite precipitates might inhibit plastic deformation by impeding the movement of dislocations through the ferrite matrix).
Rather, they increase the bulk hardness of the cast iron simply by virtue of their own very high hardness and their substantial volume fraction, such that the bulk hardness can be approximated by a rule of mixtures. In any case, they offer hardness at the expense of toughness. Since carbide makes up a large fraction of the material, white cast iron could reasonably be classified as a cermet. White iron is too brittle for use in many structural components, but with good hardness and abrasion resistance and relatively low cost, it finds use in such applications as the wear surfaces (impeller and volute) of slurry pumps, shell liners and lifter bars in ball mills and autogenous grinding mills, balls and rings in coal pulverisers, and the teeth of a backhoe’s digging bucket (although cast medium-carbon martensitic steel is more common for this application).
Malleable cast iron
Malleable iron starts as a white iron casting that is then heat treated at about 900 °C (1,650 °F). Graphite separates out much more slowly in this case, so that surface tension has time to form it into spheroidal particles rather than flakes. Due to their lower aspect ratio, spheroids are relatively short and far from one another, and have a lower cross section vis-a-vis a propagating crack or phonon. They also have blunt boundaries, as opposed to flakes, which alleviates the stress concentration problems faced by grey cast iron. In general, the properties of malleable cast iron are more like mild steel. There is a limit to how large a part can be cast in malleable iron, since it is made from white cast iron.
Ductile cast iron
A more recent development is nodular or ductile cast iron. Tiny amounts of magnesium or cerium added to these alloys slow down the growth of graphite precipitates by bonding to the edges of the graphite planes. Along with careful control of other elements and timing, this allows the carbon to separate as spheroidal particles as the material solidifies. The properties are similar to malleable iron, but parts can be cast with larger sections.
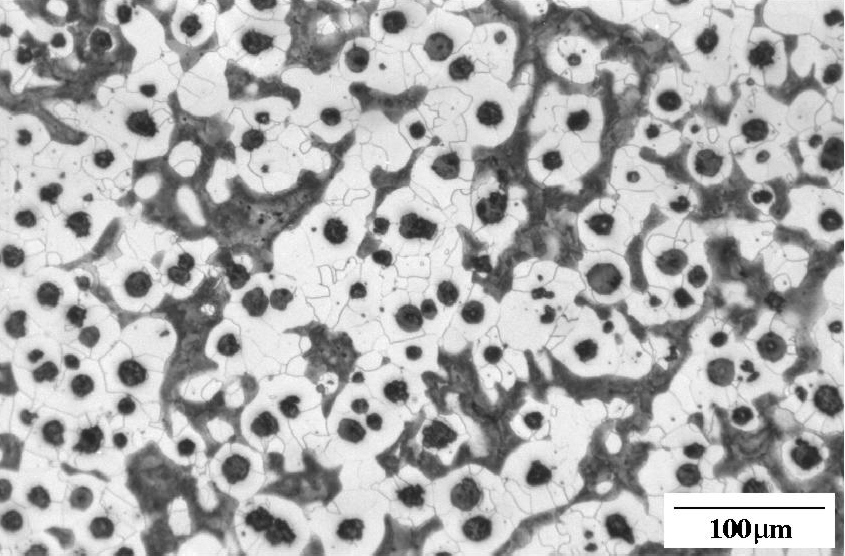 Ductile iron as-cast. Nodules of graphite, pearlite (dark islands) and ferrite (light background). Etchant: Nital 2%
Ductile iron as-cast. Nodules of graphite, pearlite (dark islands) and ferrite (light background). Etchant: Nital 2%
Cast iron’s properties are changed by adding various alloying elements, or alloyants. Next to carbon, silicon is the most important alloyant because it forces carbon out of solution. Instead the carbon forms graphite which results in a softer iron, reduces shrinkage, lowers strength, and decreases density. Sulfur, when added, forms iron sulfide, which prevents the formation of graphite and increases hardness. The problem with sulfur is that it makes molten cast iron sluggish, which causes short run defects.
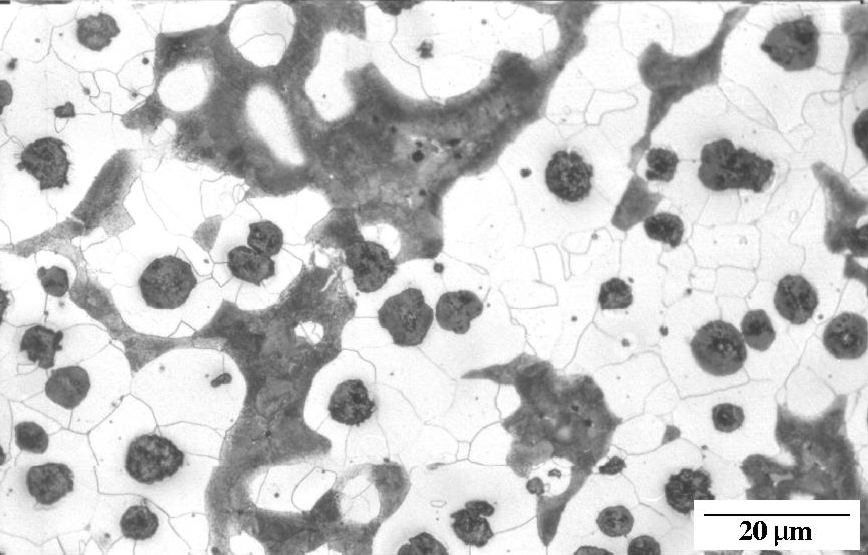 Ductile iron as-cast. Nodules of graphite, pearlite (dark islands) and ferrite (light background). Etchant: Nital 2%
Ductile iron as-cast. Nodules of graphite, pearlite (dark islands) and ferrite (light background). Etchant: Nital 2%
To counter the effects of sulfur, manganese is added because the two form into manganese sulfide instead of iron sulfide. The manganese sulfide is lighter than the melt so it tends to float out of the melt and into the slag. The amount of manganese required to neutralize sulfur is 1.7×sulfur content+0.3%. If more than this amount of manganese is added, then manganese carbide forms, which increases hardness and chilling, except in grey iron, where up to 1% of manganese increases strength and density.
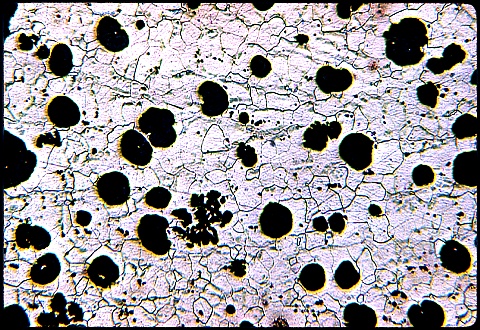 Spheroidal graphite cast iron, Fe-3.2C-2.5Si-0.05Mg wt%, containing graphite nodules in a matrix which is pearlitic. One of the nodules is surrounded by ferrite, simply because the region around the nodule is decarburised as carbon deposits on to the graphite. Etchant: Nital 2%
Spheroidal graphite cast iron, Fe-3.2C-2.5Si-0.05Mg wt%, containing graphite nodules in a matrix which is pearlitic. One of the nodules is surrounded by ferrite, simply because the region around the nodule is decarburised as carbon deposits on to the graphite. Etchant: Nital 2%
Nickel is one of the most common alloyants because it refines the pearlite and graphite structure, improves toughness, and evens out hardness differences between section thicknesses. Chromium is added in small amounts to the ladle to reduce free graphite, produce chill, and because it is a powerful carbide stabilizer; nickel is often added in conjunction. A small amount of tin can be added as a substitute for 0.5% chromium. Copper is added in the ladle or in the furnace, on the order of 0.5 to 2.5%, to decrease chill, refine graphite, and increase fluidity.
Molybdenum is added on the order of 0.3 to 1% to increase chill and refine the graphite and pearlite structure; it is often added in conjunction with nickel, copper, and chromium to form high strength irons. Titanium is added as a degasser and deoxidizer, but it also increases fluidity. 0.15 to 0.5% vanadium are added to cast iron to stabilize cementite, increase hardness, and increase resistance to wear and heat. 0.1 to 0.3% zirconium helps to form graphite, deoxidize, and increase fluidity. In malleable iron melts, bismuth is added, on the scale of 0.002 to 0.01%, to increase how much silicon can be added. In white iron, boron is added to aid in the production of malleable iron; it also reduces the coarsening effect of bismuth.









 Alloy Suppliers
Alloy Suppliers  Aluminum
Aluminum  Aluminum Extrusions
Aluminum Extrusions  Copper-Brass-Bronze
Copper-Brass-Bronze  Nickel
Nickel  Magnets
Magnets  Stainless Steel
Stainless Steel  Stainless Steel Tubing
Stainless Steel Tubing  Steel Service Centers
Steel Service Centers  Titanium
Titanium  Tungsten
Tungsten  Wire Rope
Wire Rope