Gold Electroplating - How it works ?
Gold plating is the process of adding a thin layer of gold to another metal in order to give that metal a gold-like appearance. In most cases, the layer of gold is very thin, just enough to cover all exposed surfaces of the other metal. It is commonly used in decorations and in jewelry plating.
 Gold Plating
Gold Plating
Gold plating is a method of depositing a thin layer of gold onto the surface of another metal, most often copper or silver (to make silver-gilt), by chemical or electrochemical plating. This article covers plating methods used in the modern electronics industry; for more traditional methods, often used for much larger objects, see gilding.
The benefit of gold plating for jewelry is that it gives the look of gold, but does not have the cost of gold. In jewelry applications, a gold plating is most commonly applied to silver. Thus, the jewelry actually includes two precious metals, rather than just one.
Motorola Gold Plating
While many may commonly associate gold plating with jewelry, it is not the only application for the practice. Gold plating is also commonly associated with electronic applications. This is done to allow better conductivity and make things resistant to abrasion and wear, along with other benefits.
Electroplating of Gold Plating
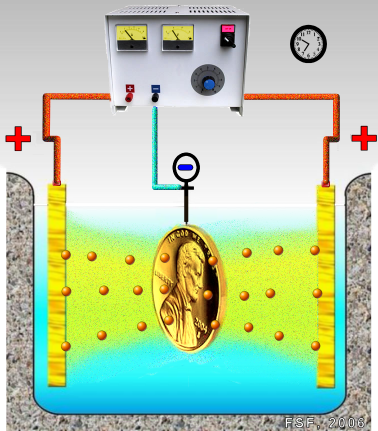
Gold plating can be accomplished in a number of different ways, including electroplating. This is done by putting the object being plated, the cathode, into a solution, usually water. It is connected to the negative side of a charge, such as a battery. Then, the donor for the plating, the anode, is also placed in the solution connected to the positive side of the charge. Ionization occurs and the anode slowly dissolves, with the positively-charged ions in the metal going to the negatively charged product.
There are several types of gold plating used in the electronics industry :
- Soft, pure gold plating is used in the semiconductor industry. The gold layer is easily soldered and wire bonded. Its Knoop hardness ranges between 60-85. The plating baths have to be kept free of contamination.
- Bright hard gold on contacts, with Knoop hardness between 120-300 and purity of 99.7-99.9% gold. Often contains a small amount of nickel and/or cobalt; these elements interfere with die bonding, therefore the plating baths can’t be used for semiconductors.
- Bright hard gold on printed circuit board tabs is deposited using lower concentration of gold in the baths. Usually contains nickel and/or cobalt as well. Edge connectors are often made by controlled-depth immersion of only the edge of the boards.
- Soft, pure gold is deposited from special electrolytes. Entire printed circuit boards can be plated. This technology can be used for depositing layers suitable for wire bonding.
There are several recognized classes of gold plating chemistry :
- Alkaline gold cyanide, for gold and gold alloy plating
- Neutral gold cyanide, for high-purity plating
- Acid gold plating for bright hard gold and gold alloy plating
- Non-cyanide, generally sulfite or chloride-based for gold and gold alloy plating
Gold plating of silver is used in the manufacture of jewelery. Like copper, silver atoms diffuse into the gold layer, causing slow gradual fading of its color and eventually causing tarnishing of the surface. This process may take months and even years, depending on the thickness of the gold layer. A barrier metal layer is used to counter this effect. Copper, which also migrates into gold, does so more slowly than silver. The copper is usually further plated with nickel. A gold-plated silver article is usually a silver substrate with layers of copper, nickel, and gold deposited on top of it.
Dubai Gold
However, gold plating, like most types of metal finishing, can be done in a number of other ways. The gold metal can be suspended in a solution, which is then brushed on the object being plated. Gold plating is often used in electronics, to provide a corrosion-resistant electrically conductive layer on copper, typically in electrical connectors and printed circuit boards. With direct gold-on-copper plating, the copper atoms tend to diffuse through the gold layer, causing tarnishing of its surface and formation of an oxide and/or sulfide layer.
Formula a gold plating solution :
- 2 real gold shares, or about 0.75 grams.
- Nitric acid (nitrate) 10 cc
- Hydrochloric acid (hydrochloric acid) 20 cc
- Sodium cyanide 20 g (or 1 cube).
- 1 liter of water.
- Plating temperature 60 degrees, or normal
- 3-12 V voltage.
- Density of current.
- The lure real gold bar.
- Colorless liquid coating characteristics (use the long yellow).
Note : If you want a gold plating solution concentration than this, add the use of gold as much as 1-2 grams.
- Gold plating solution is concentrated. Surface coating is thick, peel slowly
- The amount of nitrate and hydrochloric acid, not the exact Hai, but only the ratio of 1:2 and total amount of both acids are used to flood gold.
A layer of a suitable barrier metal, usually nickel, is often deposited on the copper substrate before the gold plating. The layer of nickel provides mechanical backing for the gold layer, improving its wear resistance. It also reduces the impact of pores present in the gold layer. Both the nickel and gold layers can be plated by electrolytic or electroless processes. There are many factors to consider in selection of either electrolytic or electroless plating methods. These include what the deposit will be used for, configuration of the part, materials compatibility and cost of processing. In different applications, electrolytic or electroless plating can have cost advantages.
At higher frequencies, the skin effect may cause higher losses due to higher electrical resistance of nickel; a nickel-plated trace can have its useful length shortened three times in the 1 GHz band in comparison with the non-plated one. Selective plating is used, depositing the nickel and gold layers only on areas where it is required and does not cause the detrimental side effects.
Wire bonding between gold plated contacts and aluminium wires or between aluminium contacts and gold wires under certain conditions develops a brittle layer of gold-aluminium intermetallics, known as purple plague. Due to the fact that gold is such a soft metal and the layer over jewelry products is so thin, there will likely be a time when the gold plating becomes worn in spots. Jewelry, especially those pieces worn daily, tend to take a substantial amount of abuse. Taking such items to a jewelry repair shop is the best way to restore them to their original luster.
You might also like
| Copper Plating How Copper Plating Works ? Electroplating... | What is Gold? Gold the Metal Non-Ferrous Gold is a chemical... | Electroplating How to make an electroplating ? Electroplating... | Microstructure of Metals Microstructure of Metals Microstructure... |


 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope