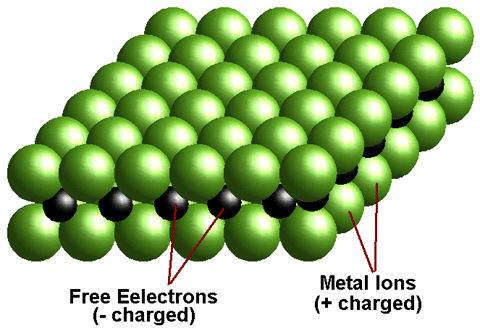
A metal is a chemical element that is a good conductor of both electricity and heat and forms cations and ionic bonds with non-metals. In chemistry, a metal is an element, compound, or alloy characterized by high electrical conductivity. In a metal, atoms readily lose electrons to form positive ions (cations). Those ions are surrounded by delocalized electrons, which are responsible for the conductivity. The solid thus produced is held by electrostatic interactions between the ions and the electron cloud, which are called metallic bonds.
A metal is defined as any element that loses its outer shell electrons to become stable. Since neutral atoms have equal numbers of electrons (- charge) and protons (+ charge). Therefore when a metal atom loses electrons it becomes positively charged. This charged particle is called an ion
Most of the elements on the periodic table are metals, including gold, silver, platinum, mercury, uranium, aluminum, sodium and calcium. Alloys, such as brass and bronze, also are metals. Metals are located on the left side and the middle of the periodic table. Group IA and Group IIA (the alkali metals) are the most active metals. The transition elements, groups IB to VIIIB, are also considered metals. The basic metals are the element to the right of the transition metals. The bottom two rows of elements beneath the body of the periodic table are the lanthanides and actinides, which are also metals.
You might also like
| Superconductors A superconductor is an element or... | Aluminum alloy Aluminum Alloy Wheel An alloy is a material... | Types of Materials Metals: Metals are elements... | Semiconductor Materials A semiconductor is a substance,... |


 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope