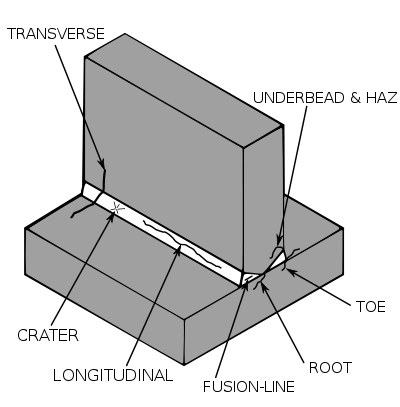
If weld preparation is good and operator induced defects (e.g. lack of penetration or fusion) are avoided, all the common structural steels can be successfully welded. However, a number of these steels may require special treatments to achieve a satisfactory joint. These treatments are not convenient in all cases.
 Welding
Welding
The difficulty in producing satisfactory welded joints in some steels arises from the extremes of heating, cooling and straining associated with the welding process combined with microstructural changes and environmental interactions that occur during welding. It is not possible for some structural steels to tolerate these effects without joint cracking occurring. The various types of cracking which can occur and the remedial measures which can be taken are discussed below.
Weld Metal Solidification Cracking
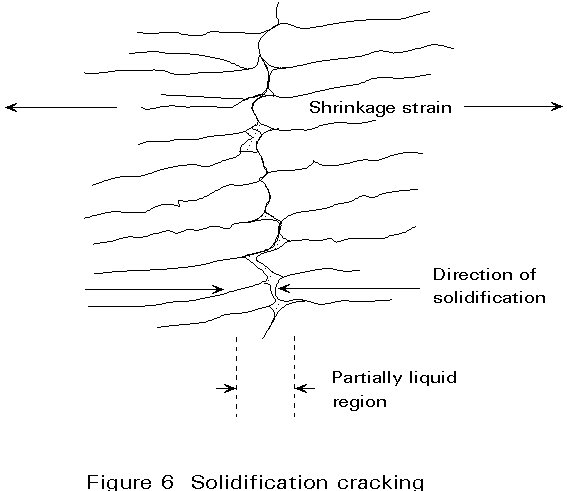
Solidification of the molten weld pool occurs by the growth of crystals away from the fusion boundary and towards the centre of the weld pool, until eventually there is no remaining liquid. In the process of crystal growth, solute and impurity elements are pushed ahead of the growing interface. This process is not significant until the final stages of solidification when the growing crystals interlock at the centre of the weld.
The high concentration of solute and impurity elements can then result in the production of a low freezing point liquid at the centre of the weld. This acts as a line of weakness and can cause cracking to occur under the influence of transverse shrinkage strains. Impurity elements such as sulphur and phosphorus are particularly important in this type of cracking since they cause low melting point silicides and phosphides to be present in the weld metal. A schematic view of solidification cracking is shown in Figure 6.
 Mig Welding
Mig Welding
Weld metals with a low susceptibility to solidification cracking (low sulphur and phosphorous) are available for most structural steels, but cracking may still arise in the following circumstances:
a. If joint movement occurs during welding, e.g. as a result of distortion. A typical example of this is welding around a patch or nozzle. If the weld is continuous, the contraction of the first part of the weld imposes a strain during solidification of the rest of the weld.
b. If contamination of the weld metal with elements such a sulphur and phosphorus occur. A typical example of this is the welding of articles with a sulphur rich scale, such as a component in a sulphur containing environment.
c. If the weld metal has to bridge a large gap, e.g. poor fit-up. In this case the depth to width ratio of the weld bead may be small. Contraction of the weld results in a large strain being imposed on the centre of the weld.
d. If the parent steel is not suitable in the sense that the diffusion of impurity elements from the steel into the weld metal can make it susceptible to cracking. Cracking susceptibility depends on the content of alloying element with the parent metal and can be expressed in the following equation:
Note: The higher the number, the greater the susceptibility.
Solidification cracking can be controlled by careful choice of parent metal composition, process parameters and joint design to avoid the circumstances previously outlined.
Heat Affected Zone (HAZ) Cracking
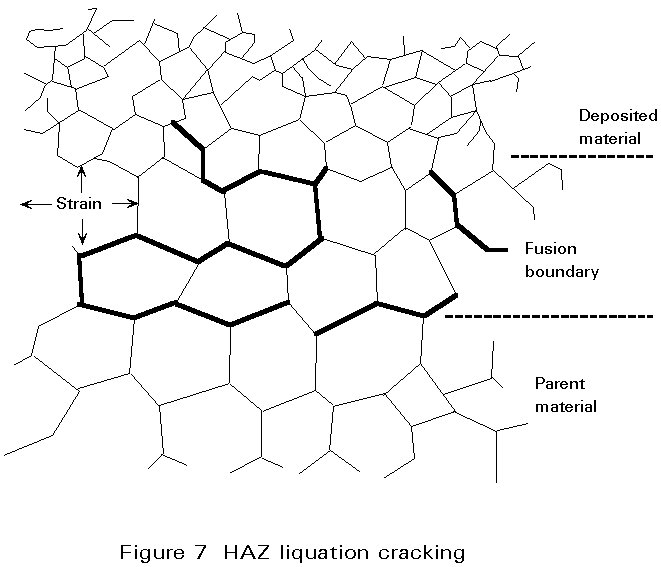
The parent material in the HAZ does not melt as a whole, but the temperature close to the fusion boundary may be so high that local melting can occur at grain boundaries due to the presence of constituents having a lower melting point than the surrounding matrix. Fine cracks may be produced in this region if the residual stress is high. These cracks can be extended by fabrication stresses or during service. A schematic view of liquation cracking is shown in Figure 7.
In steels the low melting point grain boundary films can be formed from impurities such as sulphur, phosphorus, boron, arsenic and tin. As with solidification cracking, increased carbon, sulphur and phosphorous make the steel more prone to cracking.
There are two main ways of avoiding liquation cracking. First, care should be taken to make sure that the sulphur and phosphorus levels in the parent metal are low. Unfortunately, many steel specifications permit high enough levels of sulphur and phosphorus to introduce a risk of liquation cracking. Secondly, the risk of liquation cracking is affected by the welding process used.
Processes incorporating a relatively high heat input rate, such as submerged arc or electroslag welding, lead to a greater risk of liquation cracking than, for example, manual metal arc welding. This is the case since the HAZ spends longer at the liquation temperature (allowing greater segregation of low melting point elements) and there is a greater amount of thermal strain accompanying welding.
Hydrogen induced cracking
This form of cracking (also known as HAZ, underbead, cold or delayed cracking) occurs in the HAZ at temperatures less than 200°C. Cracks can form within minutes of welding or be delayed for several days. Three factors must co-exist if cracking is to occur. These factors are:
a. The presence of hydrogen
Hydrogen is introduced into the molten weld pool during welding as a result of the decomposition of hydrogen containing compounds in the arc, e.g. moisture, grease paint and rust. Once the gas has dissolved in the weld metal, it can diffuse rapidly into the HAZ both during cooling and at ambient temperatures. In due course, the hydrogen will diffuse out of the steel. The diffusion can take a period of weeks for a thick-walled vessel.
b. A susceptible weld metal or HAZ
The cooling rate following most fusion welding processes is relatively rapid. This cooling can lead to the formation of martensite or other hardened structures in the HAZ and possibly the weld metal. These structures can be embrittled by the presence of only small quantities of hydrogen.
c. A high level of residual stress after welding
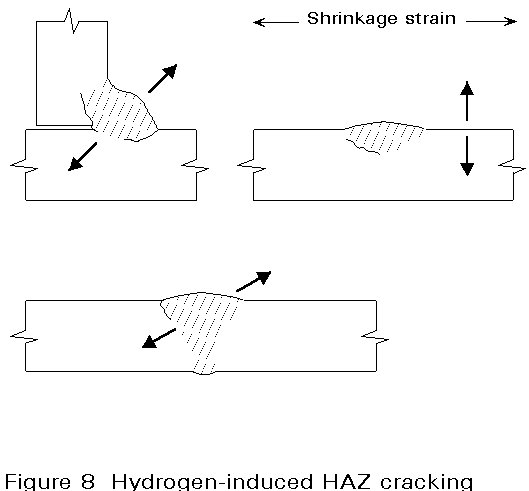
Cracking develops under the action of the residual stresses from welding in the susceptible microstructure of the HAZ or weld metal, where embrittlement has occurred due to the presence of hydrogen in solution. A schematic view of hydrogen cracking in the HAZ of different weld designs is illustrated in Figure 8.
The methods of avoiding hydrogen cracking involve removing or limiting one of the three factors which are necessary for it to occur. Hydrogen cracking can be avoided by choosing a material which does not harden in the HAZ or weld metal with the particular welding process employed. The likelihood of hardening in the HAZ is controlled by the cooling rate after welding and the hardenability of the parent steel.
 Welding Defect
Welding Defect
The hardenability of a steel is governed by its composition. A useful way of describing hardenability is to assess the total contribution to it of all the elements that are present in the steel. This assessment is done by an empirical formula which defines a carbon equivalent value (CEV) and takes account of the important elements which affect hardenability. A typical formula for the CEV (accepted in British Standards) is shown below:
As a general rule, hardening in the HAZ can be avoided by using a steel with a CEV of less than 0,42 although it should be noted that the welding process parameters influence this value. Increasing the heat input rate of the welding process (where possible) is beneficial since it results in a slower cooling rate after welding and therefore a lower likelihood of hardening in the HAZ. For the same reason, there is a less risk of hydrogen cracking when welding thin plates and sections, since the cooling rate in the HAZ is less than in thick sections.
Limiting the presence of hydrogen by avoiding damp, rust and grease, by using controlled hydrogen electrodes (properly dried basic coated electrodes) and low hydrogen welding processes (MIG or submerged arc welding) is another step towards avoiding cracking.
If these precautions are not sufficient, preheating is necessary. Preheating and the maintenance of a minimum interpass temperature during multi-pass welding has two effects. First, it results in softening of the HAZ because the cooling rate is reduced. Secondly, it accelerates the diffusion of hydrogen from the weld zone so that less remains after the weld has cooled. The minimum pre-heat temperature required to avoid hydrogen cracking depends on the chemical composition of the steel, the heat input rate and the thicknesses being joined.
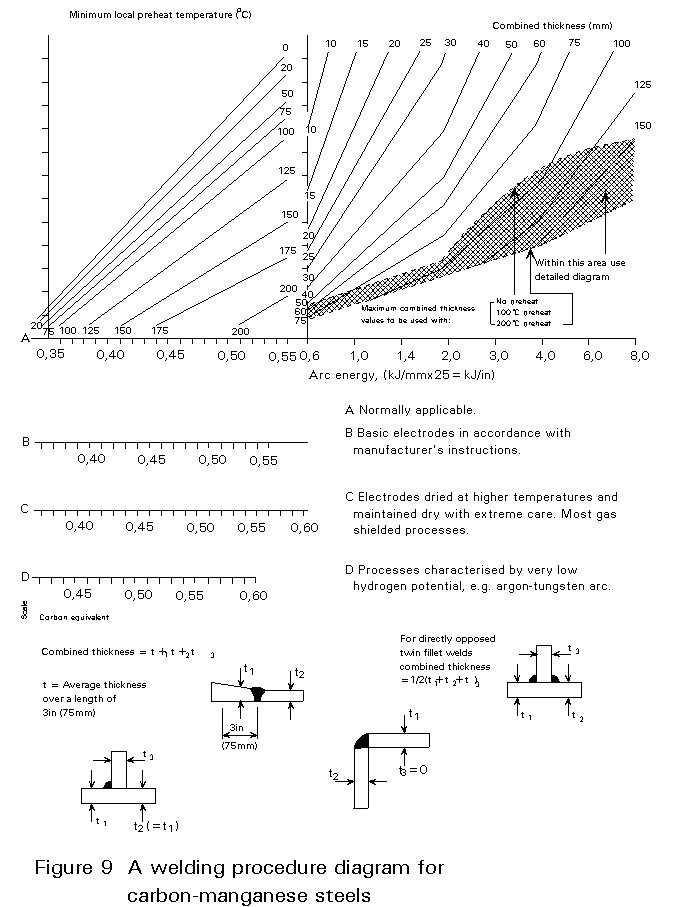
The minimum pre-heat temperature can be calculated by interrelating these facts in a welding procedure diagram. An example of one of these diagrams for carbon manganese steels is shown in Figure 9. This diagram is used in the following way :
- Select the appropriate heat input (arc energy) on the horizontal scale.
- Move vertically to intersect the appropriate combined thickness line for the joint design in question.
- Move horizontally from the intersection point to read off the pre-heat temperature for the CEV of the steel being.
Lamellar Tearing
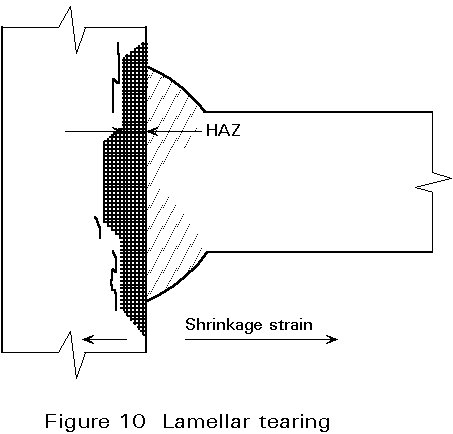
This problem can arise if the residual stresses from welding are applied across the thickness of at least one of the plates being joined. Cracking occurs if the through-thickness ductility of the plate is very low. A schematic view of this mode of cracking is shown in Figure 10.
Cracking normally occurs in the parent metal close to the outer boundary of the HAZ. The cracks have a characteristic stepped appearance with the ‘threads’ of the steps being parallel to the rolling direction of the steel plate. In contrast to hydrogen cracking, lamellar tears are not necessarily confined to the HAZ. In some cases, cracking can occur at the mid-thickness of a plate if it is restrained by a weld on both sides.
Lamellar tearing arises because the through-thickness ductility of the plate is reduced by the presence of planar inclusions lying parallel to the plate surface. All common structural steels contain large numbers of inclusions which consist of non-metallic substances produced in the steelmaking process, e.g. sulphates and silicates.
These inclusions are formed as spheres, grain boundary films, or small angular particles in the steel ingot as it cools down after casting. When the ingot is rolled to make steel plate the inclusions deform into discs parallel to the plate surface. Different types of inclusions deform in different ways and break up during rolling. The form, distribution and density of inclusions in a rolled plate determine the through-thickness ductility. Only a small proportion of steel plates have a sufficiently low through-thickness ductility to be susceptible to lamellar tearing.
Lamellar tearing can be avoided in four main ways:
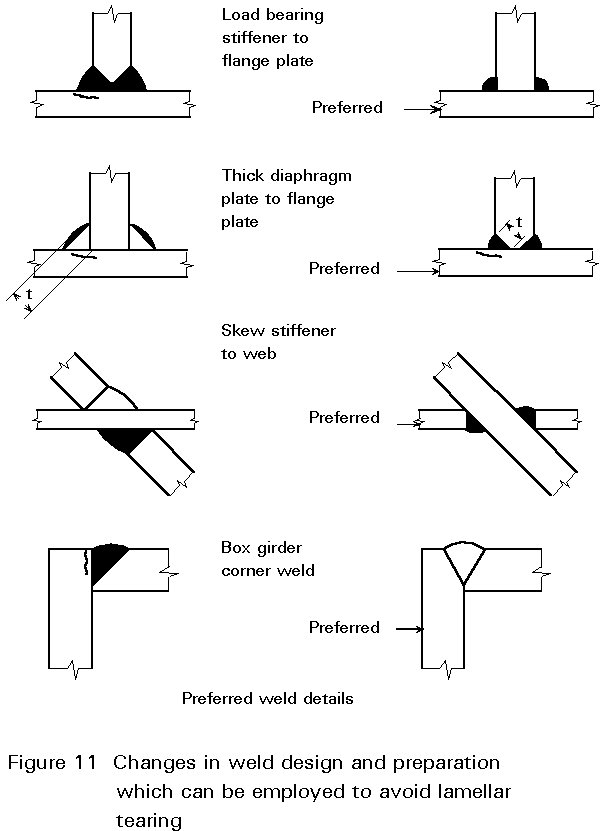
a. Improved joint design
The design of a fabrication can be altered to avoid residual stresses in the through-thickness direction of a plate. Examples are shown in Figure 11.
b. The use of forged products
The lamellar distribution of inclusions in a plate is a result of the plastic deformation occurring during rolling. The inclusion distribution in forged products is not so detrimental.
c. Plate selection
The use of steel plates with a relatively low population of planar inclusions and thus adequate through-thickness ductility.
d. Using a layer of low strength weld metal
This reduces the strain transmitted through the thickness of the welded steel plates since the soft weld metal can deform plastically. This technique, known as ‘buttering’ is relatively expensive but can be used when susceptible joints cannot be avoided.
Re-Heat Cracking
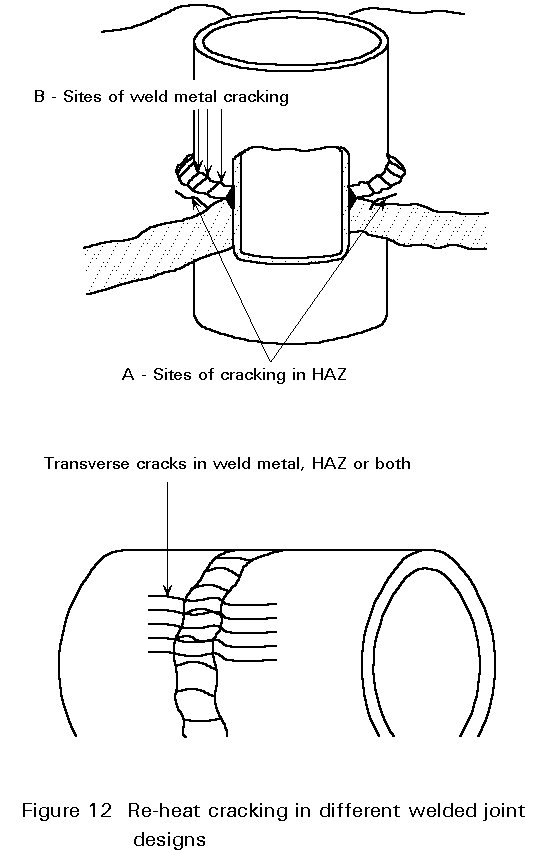
The removal or reduction of residual stresses after welding by thermal stress relief is recommended for many fabrications. In this process, the joint reaches a temperature range where rapid creep can occur (about a third to a half of the melting point). As a result, the welding residual stresses are relieved by plastic deformation. Cracking can occur during this process if the ductility of the weld or HAZ is not sufficient to accommodate the strain accompanying the residual stress relief. A schematic view of re-heat cracking is shown in Figure 12.
The residual tensile stress which acts as the driving force for the cracking process may be supplemented by transient thermal stresses in the weld zone. These stresses arise from rapid non-uniform heating up to the stress relieving temperature. The presence of geometric stress raisers, e.g. toes of fillet welds, and pre-existing cracks, e.g. liquation and hydrogen cracks, accentuate the problem.
The cracking problem is most prevalent during stress relieving operations, but it can also occur in service situations. In such cases the onset of cracking is expected to take much longer since the service temperature is generally significantly below the stress relieving temperature.
Re-heat cracking is mainly confined in practice to alloy steels containing substantial amounts of strong carbide forming elements, e.g. Cr, Mo and V. The presence of the alloy carbides inhibits grain boundary sliding and thus reduces high temperature ductility. Cracking can usually be avoided by weld profiling, e.g. grinding away any geometric stress raisers such as the toes of fillet welds, before heat treatment and by control of the heating rate to avoid high transient thermal stresses.
A structural steel can only be considered to be weldable if joints in the steel behave satisfactorily in service. In order to achieve adequate levels of performance in structural applications, the integrity of the welded joint must be good. A high level of integrity can only be achieved if the welded joint microstructure possesses sufficient ductility to resist residual stresses, which arise from the welding thermal cycle, without cracking.
The chemical compositions of both the weld and parent metals (carbon equivalent value), together with the parameters of the welding process (heat input and cooling rates), are influential in determining joint ductility.The level of impurity elements, such as sulphur, phosphorous and hydrogen, is a particularly significant factor in determining whether crack formation will occur during welding.
You might also like
| Welding Basic A Brief Description of the Welding Process Welding... | Welding Welding is a fabrication or sculptural... | Cooling Rate Cooling Rate During Austenite to Ferrite... | Rapidly Cooled Steels Formation of martensite and bainite Normalising... |
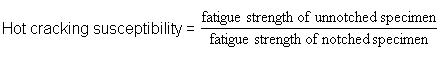
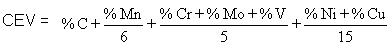



 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope