Heat Treatment
Heat Treatment is the controlled heating and cooling of metals to alter their physical and mechanical properties without changing the product shape. Heat treatment is sometimes done inadvertently due to manufacturing processes that either heat or cool the metal such as welding or forming.
Heat treating is a group of industrial and metalworking processes used to alter the physical, and sometimes chemical, properties of a material. The most common application is metallurgical. Heat treatments are also used in the manufacture of many other materials, such as glass.
Heat treatment involves the use of heating or chilling, normally to extreme temperatures, to achieve a desired result such as hardening or softening of a material. Heat treatment techniques include annealing, case hardening, precipitation strengthening, tempering and quenching. It is noteworthy that while the term heat treatment applies only to processes where the heating and cooling are done for the specific purpose of altering properties intentionally, heating and cooling often occur incidentally during other manufacturing processes such as hot forming or welding.
Heat Treatment is often associated with increasing the strength of material, but it can also be used to alter certain manufacturability objectives such as improve machining, improve formability, restore ductility after a cold working operation. Thus it is a very enabling manufacturing process that can not only help other manufacturing process, but can also improve product performance by increasing strength or other desirable characteristics.
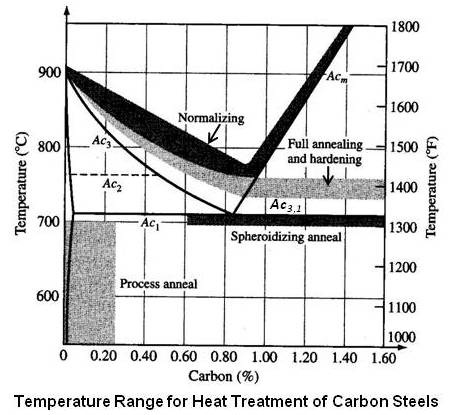
Steels are particularly suitable for heat treatment, since they respond well to heat treatment and the commercial use of steels exceeds that of any other material.
Steels are heat treated for one of the following reasons :
1. Softening
2. Hardening
3. Material Modification
Softening : Softening is done to reduce strength or hardness, remove residual stresses, improve toughnesss, restore ductility, refine grain size or change the electromagnetic properties of the steel.
Restoring ductility or removing residual stresses is a necessary operation when a large amount of cold working is to be performed, such as in a cold-rolling operation or wiredrawing. Annealing — full Process, spheroidizing, normalizing and tempering — austempering, martempering are the principal ways by which steel is softened.
Hardening : Hardening of steels is done to increase the strength and wear properties. One of the pre-requisites for hardening is sufficient carbon and alloy content. If there is sufficient Carbon content then the steel can be directly hardened. Otherwise the surface of the part has to be Carbon enriched using some diffusion treatment hardening techniques.
Material Modification : Heat treatment is used to modify properties of materials in addition to hardening and softening. These processes modify the behavior of the steels in a beneficial manner to maximize service life, e.g., stress relieving, or strength properties, e.g., cryogenic treatment, or some other desirable properties, e.g., spring aging.
Eutectoid alloys
A eutectoid alloy is similar in behavior to a eutectic alloy. A eutectic alloy is characterized by having a single melting point. This melting point is lower than that of any of the constituents, and no change in the mixture will lower the melting point any further. When a molten eutectic alloy is cooled, all of the constituents will crystallize into their respective phases at the same temperature.
A eutectoid alloy is similar, but the phase change occurs, not from a liquid, but from a solid solution. Upon cooling a eutectoid alloy from the solution temperature, the constituents will separate into different crystal phases, forming a single microstructure.
A eutectoid steel, for example, contains 0.77% carbon. Upon cooling slowly, the solution of iron and carbon, (a single phase called austenite), will separate into platelets of the phases ferrite and cementite. This forms a layered microstructure called pearlite.
Hypoeutectoid alloys
A hypoeutectic alloy has two separate melting points. Both are above the eutectic melting point for the system, but are below the melting points of any constituent forming the system. Between these two melting points, the alloy will exist as part solid and part liquid. The constituent with the lower melting point will solidify first. When completely solidified, a hypoeutectic alloy will often be in solid solution.
Similarly, a hypoeutectoid alloy has two critical temperatures, called “arrests.” Between these two temperatures, the alloy will exist partly as the solution and partly as a separate crystallizing phase. These two temperatures are called the upper (A3) and lower (A1) transformation temperatures. As the solution cools from the upper transformation temperature toward an insoluble state, the excess base metal will often be forced to “crystallize-out.” This will occur until the remaining concentration of solutes reaches the eutectoid level, which will then crystallize as a separate microstructure.
Hypereutectoid alloys
A hypereutectic alloy also has different melting points. However, between these points, it is the constituent with the higher melting point that will be solid. Similarly, a hypereutectoid alloy has two critical temperatures. When cooling a hypereutectoid alloy from the upper transformation temperature, it will usually be the excess solutes that crystallize-out first. This continues until the concentration in the remaining alloy becomes eutectoid, which then crystallizes into a separate microstructure.
A hypereutectoid steel contains more than 0.77% carbon. When slowly cooling a hypereutectoid steel, the cementite will begin to crystallize first. When the remaining steel becomes eutectoid in composition, it will crystallize into pearlite. Since cementite is much harder than pearlite, the alloy has greater hardenability at a cost in the ductility.
You might also like
| Heat Treatment Furnaces Heat Treatment Furnaces of Steel Heat treating... | Heat Treatment of Steel - the Steel Hardening Methods Heat Treatment of Metal - the Steel Hardening... | What is Stress Relieving ? Stress Relieving - A definition Stress Relieving... | What is Annealing Heat Treatment ? Annealing of Steel Heat Treatment Annealing... |



 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope