Stress Corrosion Cracking
Stress corrosion cracking (SCC) is the cracking induced from the combined influence of tensile stress and a corrosive environment. The impact of SCC on a material usually falls between dry cracking and the fatigue threshold of that material. This factor makes it common for SCC to go undetected prior to failure.
 Stress corrosion cracking
Stress corrosion cracking
SCC often progresses rapidly, and is more common among alloys than pure metals. The specific environment is of crucial importance, and only very small concentrations of certain highly active chemicals are needed to produce catastrophic cracking, often leading to devastating and unexpected failure.
Stress corrosion cracking (SCC) is a process involving the initiation of cracks and their propagation, possibly up to complete failure of a component, due to the combined action of tensile mechanical loading and a corrosive medium. Indeed, it is the presence of tensile stresses that is dangerous, compressive stresses exerting a protective influence.
Stress corrosion cracking (SCC) is the unexpected sudden failure of normally ductile metals subjected to a tensile stress in a corrosive environment, especially at elevated temperature in the case of metals. SCC is highly chemically specific in that certain alloys are likely to undergo SCC only when exposed to a small number of chemical environments.
The chemical environment that causes SCC for a given alloy is often one which is only mildly corrosive to the metal otherwise. Hence, metal parts with severe SCC can appear bright and shiny, while being filled with microscopic cracks.
Cold deformation and forming, welding, heat treatment, machining and grinding can introduce residual stresses. The magnitude and importance of such stresses is often underestimated. The residual stresses set up as a result of welding operations tend to approach the yield strength. The build-up of corrosion products in confined spaces can also generate significant stresses and should not be overlooked. SCC usually occurs in certain specific alloy-environment-stress combinations.
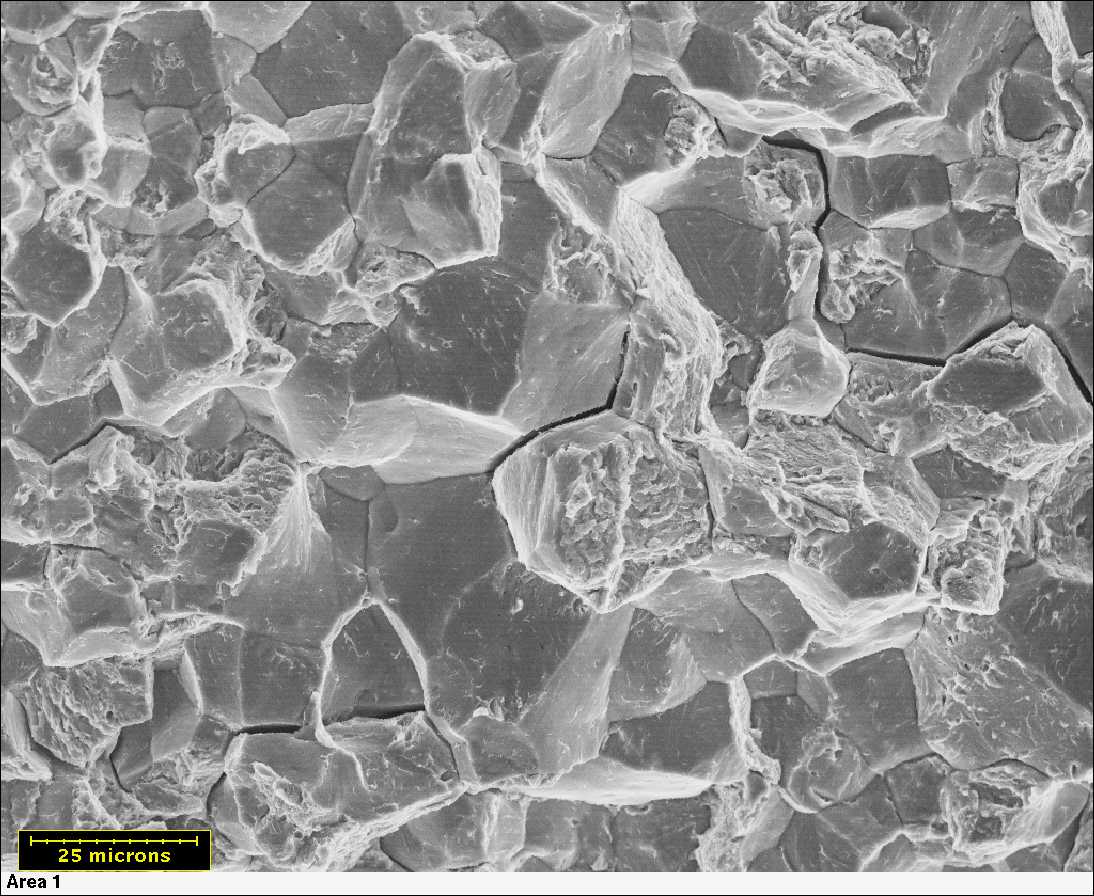
 Macroscopically, SCC fractures have a brittle appearance. Experimental SCC data is notorious for a wide range of scatter. Stress-corrosion cracking refers to cracking caused by the simultaneous presence of tensile stress and a specific corrosive medium.
Macroscopically, SCC fractures have a brittle appearance. Experimental SCC data is notorious for a wide range of scatter. Stress-corrosion cracking refers to cracking caused by the simultaneous presence of tensile stress and a specific corrosive medium.
Many investigators have classified all cracking failures occurring in corrosive mediums as stress-corrosion cracking, including failures due to hydrogen embrittlement.
To illustrate, cathodic protection is an effective method for preventing stress-corrosion cracking whereas it rapidly accelerates hydrogen-embrittlement effects. Hence, the importance of considering stress-corrosion cracking and hydrogen embrittlement as separate phenomena is obvious. During stress-corrosion cracking, the metal or alloy is virtually unattacked over most of its surface, while fine cracks progress through it. This cracking phenomenon has serious consequences since it can occur at stresses within the range of typical design stress.
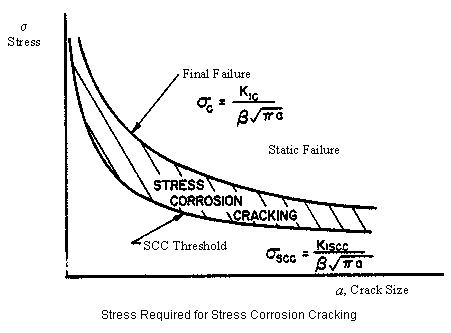
The two classic cases of stress-corrosion cracking are “season cracking” of brass, and the “caustic embrittlement” of steel. Both of these obsolete terms describe the environmental conditions present which led to stress-corrosion cracking. Season cracking refers to the stress-corrosion cracking failure of brass cartridge cases. In order for SCC to occur, we require a susceptible material, an environment that will cause cracking of that material and a high enough stress or stress intensity factor. In an ideal world a stress corrosion cracking control strategy will start operating at the design stage, and will focus on the selection of material, the limitation of stress and the control of the environment.
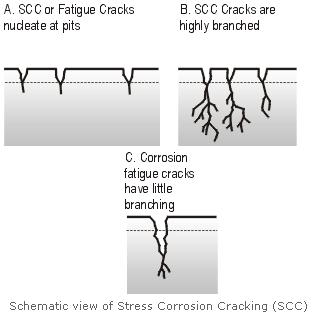 The first line of defence in controlling stress corrosion cracking is to be aware of the possibility at the design and construction stages. By choosing a material that is not susceptible to SCC in the service environment, and by processing and fabricating it correctly, subsequent SCC problems can be avoided. Some environments, such as high temperature water, are very aggressive, and will cause SCC of most materials.
The first line of defence in controlling stress corrosion cracking is to be aware of the possibility at the design and construction stages. By choosing a material that is not susceptible to SCC in the service environment, and by processing and fabricating it correctly, subsequent SCC problems can be avoided. Some environments, such as high temperature water, are very aggressive, and will cause SCC of most materials.
As one of the requirements for stress corrosion cracking is the presence of stress in the components, one method of control is to eliminate that stress, or at least reduce it below the threshold stress for SCC. This is not usually feasible for working stresses (the stress that the component is intended to support), but it may be possible where the stress causing cracking is a residual stress introduced during welding or forming.
Residual stresses can be relieved by stress-relief annealing, and this is widely used for carbon steels. These have the advantage of a relatively high threshold stress for most environments, consequently it is relatively easy to reduce the residual stresses to a low enough level. In contrast austenitic stainless steels have a very low threshold stress for chloride SCC. Stresses can also be relieved mechanically. Similarly shot-peening or grit-blasting tend to introduce a surface compressive stress, and are beneficial for the control of SCC.
The most direct way of controlling SCC through control of the environment is to remove or replace the component of the environment that is responsible for the problem. For example, chloride stress corrosion cracking of austenitic stainless steels has been experienced in hot-water jackets around chocolate pipes (that is to say, pipes carrying molten chocolate) in the food industry.
To take another example of chloride SCC of austenitic stainless steels, tube and shell heat exchangers are frequently constructed using stainless steel tubes (since these must be thin-walled and corrosion cannot be tolerated) with carbon steel tube plates and shell (since these can be made much thicker to provide a corrosion allowance). Chloride SCC is rarely experienced with this construction.
The result is frequently a rapid failure of the heat exchanger by SCC or pitting corrosion. This is because the carbon steel adopts a relatively low electrode potential that is well below that required to cause SCC or pitting of austenitic stainless steel, which is thereby protected. Indeed chemicals that inhibit general corrosion may create the necessary conditions for stress corrosion cracking (e.g. hydroxides, carbonates and nitrates for carbon steel). Metallic coatings isolate the metal from the environment, and can, thereby, prevent SCC. Cadmium adopts a rather more positive potential, and produces a much lower risk of hydrogen embrittlement, while still protecting the underlying steel.
You might also like
| Hydrogen Embrittlement Hydrogen Embrittlement - Definition and Meaning When... | Fracture Toughness Failure Analysis - Fracture Toughness Fracture... | Weldability of Structural Steels What is Weldability of Structural Steels... | Failure analysis Failure Analysis - Definition and Overview Failure... |



 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope