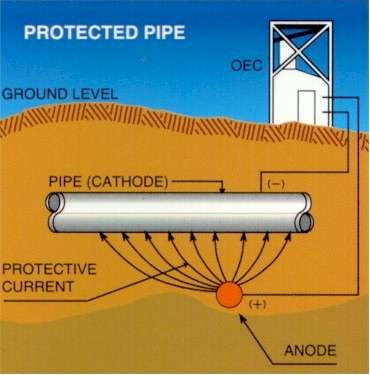
Cathodic Protection
The basic concept of cathodic protection is that the electrical potential of the subject metal is reduced below its corrosion potential, and that it will then be incapable of going into solution, or corroding.
Indeed the principles of corrosion reactions are used in the design and construction of expendable and re-chargeable batteries and accumulators which play such a major part in modern life. Likewise a car battery on charge does not corrode, in fact in this case the reaction is reversible, and energy is ‘pumped back in’.
You might also like
| Anti Corrosion What is Rust Inhibitor ? Anti-corrosion... | Zinc Alloys What is Zinc Alloy ? Zinc Alloys are combinations... | Hydrogen Embrittlement Hydrogen Embrittlement - Definition and Meaning When... | Corrosion Stainless Steel How can stainless steel corrode? All "stainless"... |













 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope