What is Anodizing ?
Anodizing is an electrochemical process that converts the metal surface into a decorative, durable, corrosion-resistant, anodic oxide finish. Aluminum is ideally suited to anodizing, although other nonferrous metals, such as magnesium and titanium, also can be anodized.
Anodizing Aluminum
Anodizing, or anodising in British English, is an electrolytic passivation process used to increase the thickness of the natural oxide layer on the surface of metal parts. The process is called “anodizing” because the part to be treated forms the anode electrode of an electrical circuit. Anodizing increases corrosion resistance and wear resistance, and provides better adhesion for paint primers and glues than does bare metal. Anodic films can also be used for a number of cosmetic effects, either with thick porous coatings that can absorb dyes or with thin transparent coatings that add interference effects to reflected light.
Anodizing is also used to prevent galling of threaded components and to make dielectric films for electrolytic capacitors. Anodic films are most commonly applied to protect aluminium alloys, although processes also exist for titanium, zinc, magnesium, niobium, and tantalum. Iron or carbon steel metal exfoliates when oxidized under neutral or alkaline microelectrolytic conditions; i.e., the iron oxide (actually “ferric hydroxide” or hydrated iron oxide, also known as rust) forms by anoxic anodic pits and large cathodic surface, these pits concentrate anions such as sulfate and chloride accelerating the underlying metal to corrosion.
Carbon flakes or nodules in iron or steel with high carbon content (high carbon steel, cast iron) may cause an electrolytic potential and interfere with coating or plating.
Ferrous metals are commonly anodized electrolytically in nitric acid, or by treatment with red fuming nitric acid, to form hard black ferric oxide. This oxide remains conformal even when plated on wire and the wire is bent.
The anodic oxide structure originates from the aluminum substrate and is composed entirely of aluminum oxide. Anodizing is accomplished by immersing the aluminum into an acid electrolyte bath and passing an electric current through the medium. An oxide film can be grown on certain metals – aluminum, niobium, tantalum, titanium, tungsten, zirconium – by an electrochemical process called anodizing. For each of these metals there are process conditions which promote growth of a thin, dense, barrier oxide of uniform thickness.
Aluminum is unique among these metals in that, in addition to the thin barrier oxide, anodizing aluminum alloys in certain acidic electrolytes produces a thick oxide coating, containing a high density of microscopic pores.
When the circuit is closed, electrons are withdrawn from the metal at the positive terminal, allowing ions at the metal surface to react with water to form an oxide layer on the metal. Bath electrolytes are selected in which the oxide is insoluble, or dissolves at a slower rate than it deposits, and then an adherent oxide layer grows.
Barrier oxide grows in near neutral solutions in which aluminum oxide is hardly soluble, most commonly ammonium borate, phosphate, or tartrate compositions. Porous oxide grows in acid electrolytes in which oxide can not only be deposited but also dissolves. Other baths used for particular applications are made with oxalic acid or phosphoric acid.
Anodization changes the microscopic texture of the surface and changes the crystal structure of the metal near the surface. Thick coatings are normally porous, so a sealing process is often needed to achieve corrosion resistance. Anodized aluminium surfaces, for example, are harder than aluminium but have low to moderate wear resistance that can be improved with increasing thickness or by applying suitable sealing substances. Anodizing most closely resembles standard electroplating. This oxygen reacts with the metal to form a thin oxide film that generates colors. This phenomenon of voltage controlled growth means that the color is also voltage controlled.
Aluminium alloys are anodized to increase corrosion resistance, to increase surface hardness, and to allow dyeing (coloring), improved lubrication, or improved adhesion.
When exposed to air at room temperature, or any other gas containing oxygen, pure aluminium self-passivates by forming a surface layer of amorphous aluminium oxide 2 to 3 nm thick, which provides very effective protection against corrosion.
Aluminium alloys typically form a thicker oxide layer, 5-15 nm thick, but tend to be more susceptible to corrosion. Aluminium alloy parts are anodized to greatly increase the thickness of this layer for corrosion resistance.
Although anodizing produces a very regular and uniform coating, microscopic fissures in the coating can lead to corrosion. Further, the coating is susceptible to chemical dissolution in the presence of high and low pH chemistry, which results in stripping the coating and corrosion of the substrate.
For instance, sulfuric anodized articles are normally sealed, either through hydro-thermal sealing or precipitating sealing, to reduce porosity and interstitial pathways that allow for corrosive ion exchange between the surface and the substrate. Some aluminium aircraft parts, architectural materials, and consumer products are anodized. Although anodizing only has moderate wear resistance, the deeper pores can better retain a lubricating film than a smooth surface would.
Anodized coatings have a much lower thermal conductivity and coefficient of linear expansion than aluminium. The coating can crack, but it will not peel. The melting point of aluminium oxide is 2050 °C, much higher than pure aluminium’s 658 °C.
In typical commercial aluminium anodization processes, the aluminium oxide is grown down into the surface and out from the surface by equal amounts. So anodizing will increase the part dimensions on each surface by half of the oxide thickness.
If the part is anodized on all sides, then all linear dimensions will increase by the oxide thickness. Anodized aluminium surfaces are harder than aluminium but have low to moderate wear resistance, although this can be improved with thickness and sealing.
An area of oxide produced with a high voltage will not pass current from a lower voltage. In other words an area anodized at 60 volts will not need masking when an adjacent area is anodized to 40 volts. Anodizing was first used on an industrial scale in 1923 to protect Duralumin seaplane parts from corrosion. This early chromic acid-based process was called the Bengough-Stuart process and was documented in British defence specification DEF STAN 03-24/3. Sulfuric acid soon became and remains the most common anodizing electrolyte.
Anodizing’s Benefits :
The unique anodized finish is the only one in the metals industry that satisfies each of the factors that must be considered when selecting a high performance aluminum finish
- Durability. Most anodized products have an extremely long life span and offer significant economic advantages through maintenance and operating savings. Anodizing is a reacted finish that is integrated with the underlying aluminum for total bonding and unmatched adhesion.
- Color Stability. Exterior anodic coatings provide good stability to ultraviolet rays, do not chip or peel, and are easily repeatable.
- Ease of Maintenance. Scars and wear from fabrication, handling, installation, frequent surface dirt cleaning and usage are virtually non-existent. Rinsing or mild soap and water cleaning usually will restore an anodized surface to its original appearance. Mild abrasive cleaners can be used for more difficult deposits.
- Aesthetics. Anodizing offers a large increasing number of gloss and color alternatives and minimizes or eliminates color variations. Unlike other finishes, anodizing allows the aluminum to maintain its metallic appearance.
- Cost. A lower initial finishing cost combines with lower maintenance costs for greater long-term value.
- Health and Safety. Anodizing is a safe process that is not harmful to human health. An anodized finish is chemically stable, will not decompose; is non-toxic; and is heat-resistant to the melting point of aluminum (1,221 degrees F.)
Since then, finishing technology has provided a steady stream of protection and coloring improvements. The first and most important of these was the development of the anodizing process, which converts the aluminum surface into an extremely hard, durable, corrosion resistant, long-lasting aluminum oxide finish.
The aluminum anodizing process today includes specialized lines, such as batch, continuous coil, continuous parts and even basket anodizing for small parts. This specialized processing technology has allowed companies who anodize to remain competitive and at the same time give excellent quality. Recent breakthroughs in coloring techniques, gives a range of colors rivaling paint, but with the technical advantages and beauty of anodizing.
You might also like
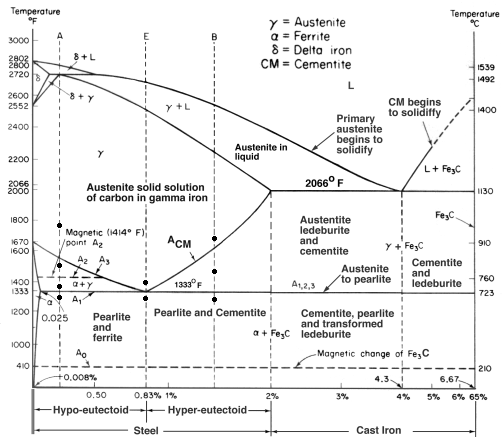
| Corrosion What is Corrosion ? Corrosion is the disintegration... | Iron Carbon Phase Diagram - Fe-Fe3C and T-T-T Diagram Iron Carbon Phase Diagram Iron-carbon... | Gold Plating Gold Electroplating - How it works ? Gold... | Hydrogen Embrittlement Hydrogen Embrittlement - Definition and Meaning When... |


 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope