What sre Eutectoid Alloys ?
A eutectoid alloy is similar in behavior to a eutectic alloy. A eutectic alloy is characterized by having a single melting point. This melting point is lower than that of any of the constituents, and no change in the mixture will lower the melting point any further. When a molten eutectic alloy is cooled, all of the constituents will crystallize into their respective phases at the same temperature.
This is unusually coarse pearlite in a eutectoid (0.8% carbon) steel
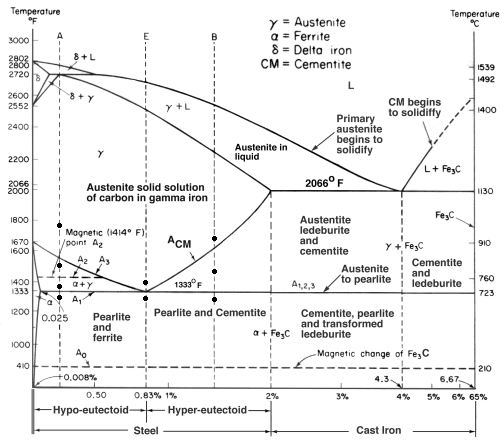
A eutectoid alloy is similar, but the phase change occurs, not from a liquid, but from a solid solution. Upon cooling a eutectoid alloy from the solution temperature, the constituents will separate into different crystal phases, forming a single microstructure. A eutectoid steel, for example, contains 0.77% carbon.
A phase diagram for a fictitious binary chemical mixture (with the two components denoted by A and B) used to depict the eutectic composition, temperature, and point. (L denotes the liquid state.)
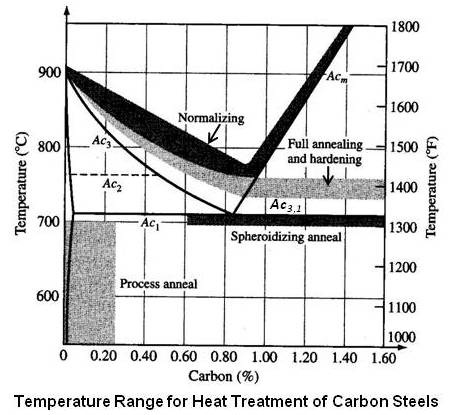
Upon cooling slowly, the solution of iron and carbon, (a single phase called austenite), will separate into platelets of the phases ferrite and cementite. This forms a layered microstructure called pearlite.
Since pearlite is harder than iron, the degree of softness achieveable is typically limited to that produced by the pearlite. Similarly, the hardenability is limited by the continuous martensitic microstructure formed when cooled very fast.
A eutectic system is a mixture of chemical compounds or elements that has a single chemical composition that solidifies at a lower temperature than any other composition made up of the same ingredients. This composition is known as the eutectic composition and the temperature is known as the eutectic temperature.
On a phase diagram the intersection of the eutectic temperature and the eutectic composition gives the eutectic point. Not all binary alloys have a eutectic point; for example, in the silver-gold system the melt temperature (liquidus) and freeze temperature (solidus) both increase monotonically as the mix changes from pure silver to pure gold.
You might also like
| Phase Diagram of Steel - Problem Solving Problem Solving at Steel Phase Diagram Iron-carbon... | Do you know Heat Treatment process ? Heat Treatment Heat Treatment is the controlled... | Metallurgy Glossary What is Metallurgy ? Metallurgy is a domain... | Iron Carbon Phase Diagram - Fe-Fe3C and T-T-T Diagram Iron Carbon Phase Diagram Iron-carbon... |

 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope