Hydrogen embrittlement
Hydrogen embrittlement is the process by which various metals, most importantly high-strength steel, become brittle and fracture following exposure to hydrogen. Hydrogen embrittlement is often the result of unintentional introduction of hydrogen into susceptible metals during forming or finishing operations.
Hydrogen embrittlement is also used to describe the formation of zircaloy hydride. Use of the term in this context is common in the nuclear industry.
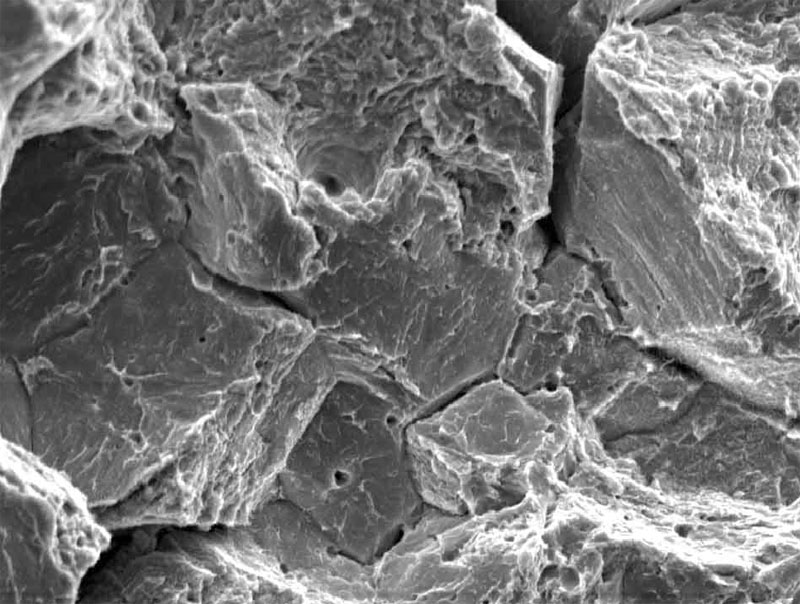 SEM image of fracture resulting from hydrogen embrittlement.
SEM image of fracture resulting from hydrogen embrittlement.
The mechanism starts with lone hydrogen atoms diffusing through the metal. At high temperatures, the elevated solubility of hydrogen allows hydrogen to diffuse into the metal (or the hydrogen can diffuse in at a low temperature, assisted by a concentration gradient). When these hydrogen atoms re-combine in minuscule voids of the metal matrix to form hydrogen molecules, they create pressure from inside the cavity they are in. This pressure can increase to levels where the metal has reduced ductility and tensile strength up to the point where it cracks open (hydrogen induced cracking, or HIC).
Hydrogen embrittlement fractures are caused by hydrogen entering the metal and concentrating internally in high-stress areas, making the metal very brittle. Hydrogen induced cracking can also occur if the metal is subjected to cyclic stresses or tensile stress.
High-strength and low-alloy steels, nickel and titanium alloys are most susceptible. Austempered iron is also susceptible.[citation needed] Steel with an ultimate tensile strength of less than 1000 MPa or hardness of less than 30 HRC are not generally considered susceptible to hydrogen embrittlement. Jewett et al. reports the results of tensile tests carried out on several structural metals under high-pressure molecular hydrogen environment. These tests have shown that austenitic stainless steels, aluminum (including alloys), copper (including alloys, e.g. beryllium copper) are not susceptible to hydrogen embrittlement along with few other metals. For example of a severe embrittlement measured by Jewett, the elongation at failure of 17-4PH precipitation hardened stainless steel was measured to drop from 17% to only 1.7% when smooth specimens were exposed to high-pressure hydrogen.
Hydrogen embrittlement can occur during various manufacturing operations or operational use - anywhere that the metal comes into contact with atomic or molecular hydrogen. Processes that can lead to this include cathodic protection, phosphating, pickling, and electroplating.
A special case is arc welding, in which the hydrogen is released from moisture (for example in the coating of the welding electrodes; to minimize this, special low-hydrogen electrodes are used for welding high-strength steels). Other mechanisms of introduction of hydrogen into metal are galvanic corrosion, chemical reactions of metal with acids, or with other chemicals (notably hydrogen sulfide in sulfide stress cracking, or SSC, a process of importance for the oil and gas industries).
You might also like
| Hydrogen Embrittlement Hydrogen Embrittlement - Definition and Meaning When... | Do you know the Stress Corrosion Cracking ? Stress Corrosion Cracking Stress corrosion... | Weldability of Structural Steels What is Weldability of Structural Steels... | Zinc Alloys What is Zinc Alloy ? Zinc Alloys are combinations... |



 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope